Top Stories
FDA Issues Urgent Botox Warning After Botulism Cases Surge
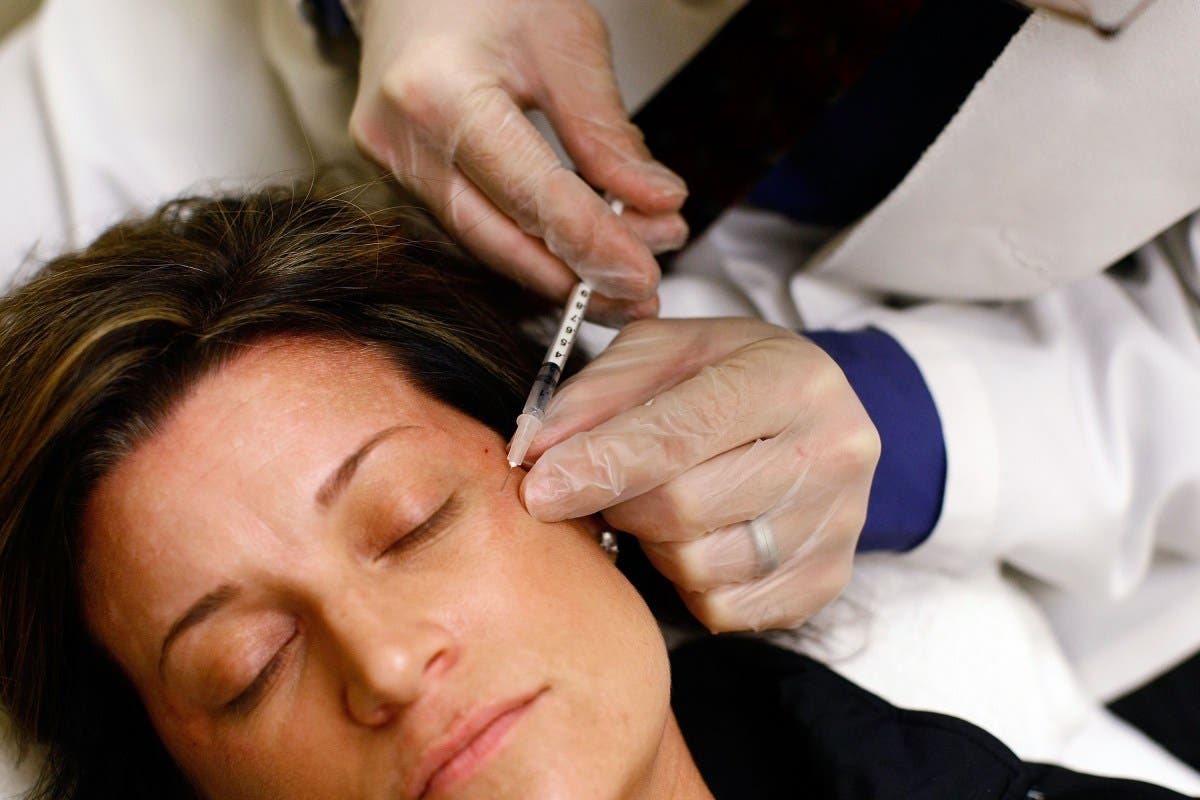
UPDATE: The U.S. Food and Drug Administration (FDA) has just issued a critical warning to halt the sale of unapproved Botox products linked to serious health risks, including a rare and potentially fatal illness known as botulism. This urgent announcement comes after reports of adverse reactions from at least 22 individuals across 11 states between November 2023 and March 2024.
The FDA’s warning emphasizes the dangers of counterfeit Botox injections, which are often marketed online without proper authorization. These dangerous products can lead to severe side effects, including muscle weakness, difficulty swallowing, and even respiratory failure. Officials have identified that the majority of affected individuals were women aged 25 to 59, with 11 requiring hospitalization due to their symptoms.
In a decisive move, the FDA sent warning letters to 18 websites accused of illegally selling unapproved and misbranded Botox. These online sellers have been flagged for posing a significant risk to public health by distributing products that have not been verified by the agency. The implicated websites include acecosm.com, aesthetic-essentials.com, and glamderma.com, among others.
“Unapproved and misbranded Botox products carry serious health risks,” said FDA Commissioner Marty Makary. He stressed that the agency is taking strong actions to protect American consumers and eliminate the dangerous sale of these products online.
Experts urge consumers to ensure that any Botox they consider is administered by licensed professionals. Oma Agbai, a board-certified dermatologist, emphasized the importance of patient advocacy in cosmetic procedures: “Patients should take an active role in advocating for their own safety.”
The investigation follows a troubling increase in reports of adverse events linked to unauthorized botulinum toxin products. The Centers for Disease Control and Prevention (CDC) has documented these cases, primarily involving cosmetic injections performed outside of licensed healthcare facilities, including homes and spas. Symptoms reported range from drooping eyelids to severe respiratory distress.
The FDA is urging anyone who has received suspicious injections to seek medical attention immediately if they experience symptoms associated with botulism, such as vision changes or difficulty breathing.
As the FDA and CDC continue to monitor the situation, consumers are advised to verify both the product and the provider before undergoing any botulinum toxin injection. This warning serves as a crucial reminder of the potential dangers associated with unregulated cosmetic procedures.
Stay informed as this developing story unfolds and ensure your safety by only opting for FDA-approved products administered by certified professionals.
-

 Sports3 weeks ago
Sports3 weeks agoSteve Kerr Supports Jonathan Kuminga After Ejection in Preseason Game
-

 Top Stories2 weeks ago
Top Stories2 weeks agoMarc Buoniconti’s Legacy: 40 Years Later, Lives Transformed
-

 Entertainment3 weeks ago
Entertainment3 weeks agoZoe Saldana Advocates for James Cameron’s Avatar Documentary
-

 Science3 weeks ago
Science3 weeks agoChicago’s Viral ‘Rat Hole’ Likely Created by Squirrel, Study Reveals
-

 Politics3 weeks ago
Politics3 weeks agoDallin H. Oaks Assumes Leadership of Latter-day Saints Church
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoKelsea Ballerini Launches ‘Burn the Baggage’ Candle with Ranger Station
-

 Business3 weeks ago
Business3 weeks agoTyler Technologies Set to Reveal Q3 2025 Earnings on October 22
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoDua Lipa Celebrates Passing GCSE Spanish During World Tour
-

 Sports3 weeks ago
Sports3 weeks agoPatriots Dominate Picks as Raiders Fall in Season Opener
-

 Health3 weeks ago
Health3 weeks agoCommunity Unites for Seventh Annual Mental Health Awareness Walk
-

 Health3 weeks ago
Health3 weeks agoRichard Feldman Urges Ban on Menthol in Cigarettes and Vapes
-

 Business3 weeks ago
Business3 weeks agoMLB Qualifying Offer Jumps to $22.02 Million for 2024