Science
Researchers Reveal How Giant Virus Manipulates Host Amoeba
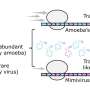
Recent research has unveiled how a giant virus creates a specialized environment within its host amoeba, enhancing its ability to replicate and become infectious. Scientists have discovered that this viral mechanism relies heavily on the intricate machinery of the host’s cellular translation system.
The study highlights the significance of codons, which are sequences of three nucleotides that guide the synthesis of proteins. The efficiency of this translation process is influenced by the compatibility of these codons with the cellular pool of transfer RNA (tRNA). When a virus utilizes rare codons that are poorly represented in the host’s tRNA pool, it often leads to ribosome pausing and instability in the messenger RNA (mRNA). This phenomenon can significantly weaken the virus’s replicative capacity.
The Role of Codons in Viral Replication
According to the research team, the choice of codons is not merely a technical detail; it plays a crucial role in the survival and infectious potential of the virus. The study reveals that by manipulating the host’s translation machinery, the virus can effectively create a “secret room” within the amoeba. This controlled environment facilitates the virus’s replication without triggering excessive immune responses from the host.
The researchers conducted experiments to demonstrate that the presence of rare codons can lead to inefficient protein production. This inefficiency often results in a decrease in viral strength, underscoring the delicate balance that viruses must maintain to thrive within their hosts.
Furthermore, understanding these mechanisms could have broader implications for virology. By identifying how viruses exploit host cellular systems, scientists can develop targeted strategies to combat viral infections. This research not only sheds light on the intricate relationship between viruses and their hosts but also opens pathways for future studies aimed at understanding viral behavior and pathogenicity.
Implications for Future Research
The findings from this research contribute significantly to our understanding of viral biology and host interactions. With the ongoing emergence of new viruses, understanding their replication strategies is essential for developing effective therapeutic interventions.
The study emphasizes the need for further investigation into the complexities of viral codon usage and its effects on translation efficiency. By delving deeper into these mechanisms, researchers hope to uncover additional strategies that viruses employ to evade host defenses, ultimately advancing our efforts in virology and infectious disease control.
This research was published in a prominent scientific journal in 2023, marking a significant step towards unraveling the complexities of viral infections. The implications of these findings will resonate within the scientific community, reinforcing the importance of understanding the fundamental processes of life at the molecular level.
-

 Science3 months ago
Science3 months agoUniversity of Hawaiʻi Joins $25.6M AI Project to Monitor Disasters
-

 Business3 months ago
Business3 months agoForeign Inflows into Japan Stocks Surge to ¥1.34 Trillion
-

 Entertainment2 months ago
Entertainment2 months agoHudson Williams Gains Popularity as Breakout Star on Heated Rivalry
-

 World3 months ago
World3 months agoBoeing’s Merger with McDonnell Douglas: A Strategic Move Explained
-

 Science2 months ago
Science2 months ago$1.25M Grant Advances Hawaiʻi’s Real-Time Hazard Monitoring
-

 Entertainment3 months ago
Entertainment3 months agoSydney Sweeney Embraces Body Positivity Amid Hollywood Challenges
-

 Top Stories3 months ago
Top Stories3 months agoBOYNEXTDOOR’s Jaehyun Faces Backlash Amid BTS-TWICE Controversy
-

 Top Stories3 months ago
Top Stories3 months agoUrgent Farewell: Joleen Chaney Leaves Legacy at KFOR
-

 World3 months ago
World3 months agoFrench Film Explores Group Therapy in ‘Group – The Schopenhauer Project’
-

 Top Stories3 months ago
Top Stories3 months agoMarc Buoniconti’s Legacy: 40 Years Later, Lives Transformed
-

 Lifestyle4 months ago
Lifestyle4 months agoKelsea Ballerini Launches ‘Burn the Baggage’ Candle with Ranger Station
-

 Top Stories3 months ago
Top Stories3 months agoCarson Wentz Out for Season After Shoulder Surgery: Urgent Update